https://doi.org/10.22319/rmcp.v14i2.6245
Article
Ixodicide action of natural products from native Mexican plants
Javier Sosa-Rueda a
Fabiola Villarauz b
Vanihamin Domínguez-Meléndez c
Ida Soto-Rodríguez b
Fernando C. López-Fentanes b
David I. Martínez-Herrera a
Álvaro Peniche-Cardeña a
Francisco Cen-Pacheco b*
a Universidad Veracruzana. Facultad de Medicina Veterinaria y Zootecnia. Miguel Ángel de Quevedo s/n, 91710, Veracruz, Veracruz, México.
b Universidad Veracruzana. Facultad de Bioanálisis. Iturbide s/n, 91700, Veracruz, Veracruz, México.
c Universidad Veracruzana. Centro de Estudios y Servicios en Salud, Veracruz, México.
* Corresponding author: fcen@uv.mx
Abstract:
This work determined the acaricidal effect of 18 Mexican plants against Rhipicephalus microplus. The results of the larvicidal assay revealed that 5 methanolic extracts produced high activity (86-100 % mortality), 3 extracts exhibited relatively high activity (71-85 % mortality), 2 extracts displayed moderate activity (56-70 % mortality), 2 extracts presented low activity (31-55 % mortality) and 6 extracts showed non-significant acaricidal activity (0-30 % mortality). Extracts inducing >56 % mortality were subsequently assayed against engorged ticks of R. microplus by adult immersion test at a concentration of 5.0% w/v. In general terms, the results on larvae and adult ticks indicated that the methanolic extracts of Annona globiflora, Annona scleroderma, Litchi chinensis and Azadirachta indica showed the greatest activities. The crude extract of A. indica was subjected to chromatographic purification, which has led to the isolation of 3-O-butyl-(-)-epigallocatechin (1), 3-O-butyl-(-)-epicatechin (2), (-)-epigallocatechin (3), (+)-gallocatechin (4), (-)-epicatechin (5), β-sitosterol (6), stigmasterol (7), stigmasterol glucoside (8), triolein (9), azadirachtin A (10), and the octadecanoic acid-tetrahydrofuran-3,4-vinyl ester (11). The isolated compounds' chemical structures were identified by the interpretation of NMR and HRESI-MS spectroscopic data. The isolated compounds were assayed against engorged ticks of R. microplus at a concentration of 6 mM. Based on the results obtained, it was concluded that 3-O-butyl-(-)-epigallocatechin (1), 3-O-butyl-(-)-epicatechin (2), azadirachtin A (10), and octadecanoic acid-tetrahydrofuran-3,4-vinyl ester (11) show the highest effectiveness.
Keywords: Mexican plants, Acaricidal screening, Azadirachta indica, Ixodicide metabolites.
Received: 24/05/2022
Accepted: 29/11/2022
Introduction
Rhipicephalus (Boophilus) microplus (R. microplus), is distributed in tropical and subtropical latitudes worldwide and is responsible for severe economic losses in livestock farming in countries in America, Africa, Asia, and Australia(1). In México, R. microplus is widely distributed, infesting several host species(2). This ectoparasite produces smaller weight gain and reduction of milk production, anemia, hide damage, and even mortalities in cattle. It is also an important vector of pathogens such as Babesia bovis, B. bigemina and Anaplasma marginale(3). Currently, tick control mostly consists of the use of acaricides, tick-resistant animals, anti-tick vaccines, and biological control(4). Among them, acaricides are the most common control method since it offers quick and cost-effective suppression of tick populations. However, the indiscriminate use of chemical substances (synthetic pyrethroids, organophosphates, macrocyclic lactones and amidines) to control tick plagues has promoted multi-resistance in this ectoparasite(5-7). Further, the accumulation of pesticides in animal tissues causes human exposure through the consumption of derived animal products(8). Therefore, it is necessary the development new substances with novel mechanisms of action and/or less toxic than those currently used. In this sense, natural products emerge as an eco-biological alternative for tick control due to their low costs and toxicity(9-12).
The chemistry of natural products has been one of the sources of inspiration for the development of new drugs over many decades, either directly as drugs or as lead structures that were further optimized by medicinal chemists(13,14). Within the wide range of natural sources, traditional herbal medicine has been one of the most prolific producers of bioactive metabolites. Indeed, phytochemical studies of medicinal plants have led to the development of over 50 % of the active pharmaceutical ingredients that are currently marketed(15-17). Azadirachta indica A. Juss, commonly known as the “neem” tree in Latin America, is a well-known curative plant with a wide range of pharmacological activities and beneficial health properties(18-20). A variety of metabolites with high structural diversity have been isolated from the “neem” tree, some of which have shown important bioactivities, like antioxidant, cytotoxic, bactericide or larvicide effects(21-24). This study evaluates the acaricidal activity of 18 extracts from Mexican plants, against larvae and engorged ticks of R. microplus. Additionally, a phytochemical study into the bark of A. indica, collected in spring 2018 in Veracruz state (México), led to the isolation of 11 natural metabolites from the “neem” tree. Their structures were determined based on detailed spectroscopic studies. The isolated compounds were evaluated against engorged female ticks.
Material and methods
Plant material
Eighteen (18) plant species were collected in spring 2018 in the region of Sotavento of Veracruz State, Mexico. Taxonomists of the Institute for Biological Research (CIB) of Veracruz University identified the plants (Table 1). After collection, the plant material was dried at room temperature for two weeks and then triturated.
Table 1: Plant species studied
Plants | Part used | Voucher | Geographic coordinates |
Annona globiflora | Seeds | 10750UV | 19º 42’ 42.6’’ N, 96º 28’ 7.2’’ W |
Annona scleroderma | Seeds | 23839UV | 19º 9’ 39.4’’ N, 96º 13’ 5.7’’ W |
Litchi chinensis | Seeds | 23764UV | 19º 10’ 26.8’’ N, 96º, 13’ 27.3’’ W |
Inga jinicuil | Seeds | 11859UV | 19º 29’ 15.8’’ N, 96º 5’ 26.5’’ W |
Ensete ventricosum | Seeds | 11244UV | 18º 17’ 15.1’’ N, 95º 18’ 51.3’’ W |
Azadirachta indica | Bark | 23765UV | 19º 10’ 26.4’’ N, 96º 13’ 22.8’’ W |
Salvia hispanica | Seeds | 11164UV | 18º 38’ 3’’ N, 97º 0’ 45’’ W |
Sterculia apetala | Seeds | 11165UV | 18º 17’ 15.1’’ N, 95º 18’ 51.3’’ W |
Citrus sinensis | Rind | 10500UV | 18º 39’ 39’’ N, 96º 56’ 18’’ W |
Citrus paradisi | Rind | 23762UV | 19º, 10’, 26.6’’ N, 96º 13’ 27.3’’ W |
Citrus latifolia | Rind | 23766UV | 19º, 10’ 30.5’’ N, 96º 13’ 28.8’’ W |
Citrus medica | Rind | 23768UV | 19º 33’ 50.4’’ N, 96º 56’ 27.6’’ W |
Mimosa pudica | Whole plant | 12879UV | 18º 3’ 50.5’’ N, 94º 22’ 13.4’’ W |
Heliotropium indicum | Whole plant | 21157UV | 18º 34’ N, 95º 4’ W |
Momordica charantia | Whole plant | 12161UV | 19º 52’ 47’’ N, 96º 67’ 86’’ W |
Tagetes erecta | Whole plant | 20090UV | 19º 43’ 50.3’’ N, 96º 43’ 40.7’’ W |
Tridax procumbens L. | Whole plant | 21537UV | 19º 18’ 29’’ N, 96º 22’ 14’’ W |
Randia aculeata | Roots | 20326UV | 19.3º 41’ 10.1’’ N, 96.3º 8’ 36.9’’ W |
Plant extraction
Plant material was extracted four times by cold maceration for 3 h at room temperature using 1 L of methanol for 300 g of plant material, each time. Afterward, the solvent was removed in vacuum in a rotary evaporator (Buchi Rotavapor R-3, Switzerland).
Isolation procedure of the compounds
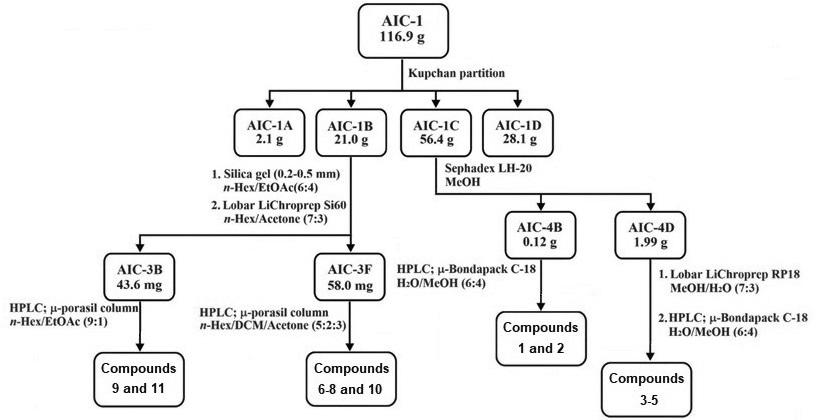
The methanolic extract of A. indica (117 g, 2.8 % dry weight) was fractionated by liquid-liquid extraction following the Kupchan method. Briefly, the extract was suspended in a methanol/water mixture (MeOH/H2O; 1 L, 1:1) and was successively separated with hexane (Hex; 3 × 1 L), dichloromethane (DCM; 3 × 1 L), and ethyl acetate (EtOAc; 3 × 1 L) (Sigma-Aldrich, St. Louis Mo., USA) to obtain four fractions of increasing polarity(25-27). The dichloromethane fraction (21.0 g) was subjected to silica gel 60 column chromatography (5 cm of internal diameter and 35 cm of length) (Merck, Darmstadt, Germany) with Hex:EtOAc (6:4), and, subsequently, in a medium-pressure Lobar LiChroprep-Si60 column (Merck, Darmstadt, Germany) with Hex:A cetone (7:3) as the eluent. The fractions collected between 6-11 min and 108-175 min were pooled together (3B and 3F, 44 and 58 mg, respectively). Final purification was performed on an HPLC with a µ-Porasil column (Waters, Wexford, Ireland), using Hex/DCM/Acetone (5:2:3) as eluent to afford β-sitosterol (6) (32.9 mg) stigmasterol (7) (39.7 mg), stigmasterol glucoside (8) (9.9 mg) and azadirachtin A (10) (28.4 mg) in fraction 3F. On the other hand, for fraction 3B, Hex/EtOAc (9:1) was used to yield trilinolein (9) (9.3 mg) and octadecanoic acid-tetrahydrofuran-3,4-vinyl ester (11) (21.7 mg). The ethyl acetate fraction (56.4 g) was chromatographed using Sephadex LH-20 column (5 × 35 cm; eluent: MeOH) (Merck, Darmstadt, Germany). The second fraction (4B 120 mg) was purified on an HPLC with a µ-BondapakTM C-18 (1.9 × 15 cm) (Waters, Wexford, Ireland) column using MeOH/H2O (2:3) to yield 3-O-butyl-(-)-epigallocatechin (1) (23.3 mg) and 3-O-butyl-(-)-epicatechin (2) (24.7 mg). The 4D fraction (2.0 g) was processed by medium-pressure chromatography, using Lobar LiChroprep-RP18 (eluent: MeOH/H2O (7:3)). Finally, HPLC was performed on a µ-BondapakTM C-18 column using MeOH/H2O (2:3) to provide three pure compounds (-)-epigallocatechin (3) (34.8 mg), (+)-gallocatechin (4) (27.9 mg) and (-)-epicatechin (5) (25.7 mg) (Figures 1 and 2).
Figure 1: Isolation procedure followed for compounds 1-11
Figure 2: Metabolites 1-11 from Azadirachta indica. In pink: the introduction of an O-butyl ether group, at C-2, in flavonoids 1 and 2 showed the best acaricidal activities
General experimental chemical procedures
NMR spectroscopy was performed on Bruker AVANCE 600 MHz instruments using CDCl3 and CD3OD at 298 K. The NMR data were acquired using standard pulse sequences. NMR data were processed using MestReNova software (v 11.01, Santiago de Compostela, Spain). HPLC separations were carried out with the HPLC Breeze 2 system (Waters, Wexford, Ireland) equipped with a UV detector. All of the solvents used were HPLC-grade. HPLC was monitored by thin layer chromatographic (TLC), performed on AL Si gel Merck 60 F254 (Kenilworth, NJ, USA). TLC plates were visualized by UV light (365 nm) and phosphomolybdic acid solution of 10 % wt in ethanol.
Tick collection
One thousand engorged females of R. microplus were collected from six naturally infested cattle on a farm located in the municipality of Puente Nacional, Veracruz, México (19°19’N; 96°28’W). These cattle had not been treated with acaricides for 45 d before the collection of ticks. Seven hundred (700) engorged females were used in the adult immersion test, and three hundred were placed in Petri dishes and incubated at 28 °C and 80 % relative humidity for two weeks to provide optimal conditions for oviposition. After, the eggs were mixed and transferred to twenty 10-mL glass vials closed with a swab of cotton for approximately 30 d, at 28 °C and 80 % relative humidity(28).
Preparation of control solutions
For the positive control, the commercial compound Taktic® (12.5%; Intervet, Mexico) was used to prepare a discriminatory dose of amitraz at 0.0002%. For the negative control, an aqueous solution with 1.0% ethanol and 0.02% Triton X-100 was prepared(29). In both cases, the final volume used was 750 μL.
Concentration of the tested samples
Methanolic extracts of the 18 plants were assayed at the concentration of 5.0% w/v (37.5 mg in 750 μL for larvae assay and 250 mg in 5 mL for adult assay). For the bio-guided purification of A. indica were used different concentrations (≤ 5.0% w/v), depending on the degree of purity of the fraction to be tested. On the other hand, compounds 1-11 were tested at a concentration of 6 mM. The final volume used in the larval immersion test was 750 μL, whereas in the adult immersion test it was 5 mL(24).
Larval immersion test
Approximately 100 larvae of R. microplus were immersed for 10 min in 750 μL of each dilution to be tested using paintbrushes. Then, they were placed on filter paper envelopes and kept at 28 °C and 80 % relative humidity for 24 h. The control group was treated with 1.0% ethanol and 0.02% Triton X-100 aqueous solution. After 24 h, dead and alive larvae were registered, and mortality percentages were calculated(30). One experiment with three replicates was used for each test.
Adult immersion test
Ten engorged female ticks with homogeneous weights (approximately 200 ± 20 mg each) were immersed for 10 min in 5 mL final volume of each solution to be tested and then dried on Whatman nº 1 filter paper. The ticks were placed in Petri dishes and kept at 28 °C and 80 % relative humidity for 24 h. After a week, the numbers of live or dead engorged females were recorded and mortality percentages calculated. One experiment with three replicates was used for each test as well as for the negative control (1.0% ethanol and 0.02% Triton X-100 solution)(30,31).
Determination of acaricidal activity
The larval mortality was corrected using Abbott’s formula as recommended by the FAO(32). Thus, the corrected mortality (CM) was calculated as follows: CM = [(test mortality % − control mortality %)/100 − control mortality %] × 100, if the mortality in control was above 7 %, the bioassay test was annulled and repeated.
In this study, acaricidal activity of the extracts was classified as follows: High: (86-100 % mortality); relatively high: (71-85 % mortality); moderate: (56-70 % mortality); low: (31-55 % mortality); and non-significant: (0-30 % mortality)(33-35).
Results
To explore the acaricidal potential of 18 Mexican plants, initially, their methanolic extracts were assessed for larvicidal activity at a cut-off concentration of 5.0 % w/v. The results revealed that 5 extracts produced high activity (86-100 % mortality), 3 extracts exhibited relatively high activity (71-85 % mortality), 2 extracts displayed moderate activity (56-70 % mortality), 2 extracts presented low activity (31-55 % mortality) and 6 extracts showed non-significant acaricidal activity (0-30 % mortality). Extracts inducing >56 % mortality were subsequently assayed against engorged ticks of R. microplus by adult immersion test at a concentration of 5.0 % w/v. These results showed that Annona globiflora, Annona scleroderma, Litchi chinensis and Azadirachta indica have the best activities in both larvae and adult ticks (Table 2). A bio-guided purification was carried out to identify the active principles responsible for the acaricidal activity in the methanolic extract of A. indica. Initially, the results of the larvicidal assay of the Kupchan fractions, Hex, DCM, EtOAc, and MeOH/H2O, showed that the activity was predominantly found in the DCM and EtOAc fractions. Thus, a chromatographic study was performed on them to identify the active compounds. Six metabolites were found from the dichloromethane fraction: β-sitosterol (6), stigmasterol (7), stigmasterol glucoside (8), trilinolein (9), azadirachtin A (10), and the octadecanoic acid-tetrahydrofuran-3,4-vinyl ester (11). In addition, 3-O-butyl-(-)-epigallocatechin (1), 3-O-butyl-(-)-epicatechin (2), (-)-epigallocatechin (3), (+)-gallocatechin (4), (-)-epicatechin (5) were isolated from the ethyl acetate fraction (Figure 2).
Table 2: Acaricidal effect of the extracts from Mexican plants at a cut-off concentration of 5.0% w/v
Plant | Key | Larva mortality (%) | Adult mortality (%) |
Annona globiflora | AGS | 100 | 100 |
Annona scleroderma | ASS | 100 | 100 |
Litchi chinensis | LCS | 91.6 ± 2.2 | 66.7 ± 5.8 |
Inga jinicuil | IJS | 89.3 ± 4.8 | 26.7 ± 15.3 |
Ensete ventricosum | EVS | 41.5 ± 13.7 | NT |
Azadirachta indica | AIC | 84.9 ± 4.3 | 53.3 ± 11.5 |
Salvia hispanica | SHS | 32.7 ± 12.4 | NT |
Sterculia apetala | SAS | 1.4 ± 2.5 | NT |
Citrus sinensis | CSR | 74.3 ± 7.9 | 10 ± 10 |
Citrus paradisi | CPR | 84.4 ± 7.1 | 3.3 ± 5.8 |
Citrus latifolia | CLR | 89.3 ± 4.2 | 16.7 ± 11.5 |
Citrus medica | CMR | 56.7 ± 9.2 | 6.7 ± 5.8 |
Mimosa pudica | MPW | 58.5 ± 9.1 | 0 |
Heliotropium indicum | HIW | 0 | NT |
Momordica charantia | MCW | 3.5 ± 6.1 | NT |
Tagetes erecta | TEW | 0.3 ± 0.4 | NT |
Tridax procumbens L. | TPW | 0 | NT |
Randia aculeata | RAR | 1.1 ± 1.0 | NT |
Amitraz1 | - | 63.2 ± 5.5 | 56.7 ± 5.8 |
± Standard deviation. 1Tested at the concentration of 0.0002%. NT: not tested.
Regarding the acaricidal assay of the isolated compounds on engorged female ticks, the results indicated that the flavonoids 3-O-butyl-(-)-epigallocatechin (1) and 3-O-butyl-(-)-epicatechin (2) caused mortality (36.7 and 43.3 %, respectively), while the other evaluated flavonoids, 3-5, showed no activity at the concentration of 6 mM. In the same way, the compounds azadirachtin A (10) (66.7 %) and the octadecanoic acid-tetrahydrofuran-3,4-vinyl ester (11) (46.7%) show good efficacy (Table 3). Finally, it was observed that compounds 3-7 did not induce adulticidal activity. Compounds 8 and 9 could not be tested, due to a lack of sample material.
Table 3: Acaricidal activity of compounds 1-11
Compound | Adult mortality (%) | Compound | Adult mortality (%) |
1 | 36.7 ± 5.8 | 7 | 0 |
2 | 43.3 ± 5.8 | 8 | NT |
3 | 0 | 9 | NT |
4 | 0 | 10 | 66.7 ± 5.8 |
5 | 0 | 11 | 46.7 ± 5.8 |
6 | 0 | Amitraz1 | 56.7 ± 5.8 |
± Standard deviation. Compounds were tested at a concentration of 6 mM.
1Tested at the concentration of 0.0002%. NT= not tested.
Discussion
Identifying the trusted range of the assay is essential to the conclusions of this investigation, especially when the resistance of ticks does not depend only on intrinsic factors such as their genetics and physiology, but also on the biotic and abiotic factors at the time of collection. That is why the standard error (SE) analysis (supplementary material) was performed, in which a variation ≤2.8 % was observed in the five extracts that generate high mortality (86-100 %). However, the SE becomes inversely proportional to mortality, that is, the less activity the standard error increases. The SE analysis suggests that the variation in future evaluations of the extracts of the 18 plants examined will be less than 5% at a concentration with high mortality (Figure 3).
Figure 3. Standard error of the larvicidal activity of the most active methanolic extracts
In general terms, the combined results of the adulticidal and larvicidal activity indicated that the methanolic extracts of Annona globiflora, Annona scleroderma, Litchi chinensis and Azadirachta indica have the best effectiveness. Annona genus has become a prolific producer of interesting compounds with great biological activity(36,37). The acaricidal activities of the extracts of A. globiflora and A. scleroderma are possibly related to the presence of acetogenins, the main chemical constituents of the Annonaceae family, which have been found to have potent pesticidal activity against a variety of arthropods(38). These results agree with the larvicidal activity reported for ethanolic extracts of the seeds of A. squamosa against R. microplus(39). In the case of the seeds of L. chinensis, previous studies have shown that this plant displays significant antimicrobial, antioxidant, and anticancer activities(40), though this is the first report of its acaricidal activity. A large number of compounds with significant structural and pharmacological diversity have been identified from A. indica. However, the acaricidal activity of the “neem” tree has been attributed to the presence of azadirachtin A (10), although there are some reports that contradict the above(41,42). Eleven major compounds were isolated from the stem bark of Azadirachta indica, among which is the azadirachtin A (10). The acaricidal assay by adult immersion test of these compounds revealed that, as well as azadirachtin A (10), some other compounds showed an acaricidal effect, such as 3-O-butyl-(-)-epigallocatechin (1), 3-O-butyl-(-)-epicatechin (2) and octadecanoic acid-tetrahydrofuran-3,4-vinyl ester (11).
About flavonoids 1-5, the results indicated that only the flavonoids 3-O-butyl-(-)-epigallocatechin (1) and 3-O-butyl-(-)-epicatechin (2) caused mortality (36.7 and 43.3%, respectively) at a concentration of 6 mM. Based on these results, it seems clear that the butyl ether fragment is essential for the activity of 1 and 2. Such butyl ethers fragments lead to an increase in the liposolubility properties of these metabolites concerning structurally-related compounds 3-5. In effect, lipophilicity is one of the most important physical properties in drug discovery, since it intervenes in the pharmacodynamics, pharmacokinetics, and toxicity of many compounds(43). For example, Echeverría et al(44) and Cen-Pacheco et al(24) reported that the bactericide and acaricidal activity of the flavonoids is associated with a narrow range of lipophilicity values (LogP between 1.5 and 3.0).
Conclusions and implications
The evaluation of 18 Mexican plants against larvae and adult ticks of R. microplus indicated that the methanolic extracts of A. globiflora, A. scleroderma, L. chinensis, and A. indica or their mixtures have great potential to be used as an alternative in the control of R. microplus. In general, the pesticide activities of A. indica are associated with azadirachtin A (10), which is the best-known insecticidal compound of this plant. However, here was report three new adulticidal compounds of the “neem” tree identified as 3-O-butyl-(-)-epigallocatechin (1), 3-O-butyl-(-)-epicatechin (2), and octadecanoic acid-tetrahydrofuran-3,4-vinyl ester (11), the above indicates that the bark of A. indica is a great source of acaricidal compounds with great structural diversity. The use of these compounds may represent a new strategy for the control of R. microplus zoonoses. From the chemical point of view, it is also important to highlight that 1 and 2 have a butyl ether group which, in addition to being uncommon in nature, increases liposolubility and their acaricidal activity.
Acknowledgments
This work was supported by the Gobierno del estado de Veracruz de Ignacio de la Llave and by the Consejo Veracruzano de Investigación Científica y Desarrollo Tecnológico ‒ [COVEICyDET, grant number 14 1953/2021]. J.S.R. thanks the CONACyT foundation for a grant (1075240). To Dr. Fernando Nicolalde-Morejón for the identification of the plants.
Conflict of interest
The authors declare no conflict of interest.
Literature cited: