https://doi.org/10.22319/rmcp.v13i3.6039
Article
Establishment of tropical forage grasses in the Cerrado biome
Antonio Leandro Chaves Gurgel a*
Gelson dos Santos Difante a
Carolina Marques Costa a
João Virgínio Emerenciano Neto b
Gustavo Henrique Tonhão a
Luís Carlos Vinhas Ítavo a
Alexandre Menezes Dias a
Iuri Mesquita Moraes Vilela a
Vivian Garcia de Oliveira a
Pâmella Cristina da Silva Lima a
Andrey William Alce Miyake a
a Universidade Federal de Mato Grosso do Sul, Faculdade de Medicina Veterinária e Zootecnia. Avenida Senador Filinto Müler, 2443 - Pioneiros, 79074-460, Campo Grande, Mato Grosso do Sul, Brasil.
b Universidade Federal do Rio Grande do Norte, Unidade Acadêmica Especializada em Ciências Agrárias. Macaíba, Rio Grande do Norte, Brasil.
* Corresponding author: antonioleandro09@gmail.com
Abstract:
This study was carried out to evaluate the time for the establishment of tropical forage grasses in the “Cerrado” biome, based on morphogenetic and structural traits. Three Brachiaria brizantha (Syn. Urochloa brizantha) cultivars (Paiaguás, Ipyporã and Marandu) and two Panicum maximum (Syn. Megathyrsus maximus) cultivars (Quênia and Tamani) were distributed in a randomized-block design with four replicates. Morphogenetic and structural traits of the pasture were assessed from d 35 to d 65 after sowing, at seven-day interval. Canopy height rose linearly with the establishment period, in all cultivars. In the Megathyrsus cultivars, tiller density decreased as the experimental period progressed, whereas the number of tillers in the Urochloa cultivars increased. The cultivars Ipyporã and Marandu had the highest leaf appearance rates. The lowest leaf elongation rates occurred in the cultivars Paiaguás, Ipyporã and Tamani, and the highest elongation rates in cv. Quênia. As a result, cv. Quênia showed the highest values of final leaf length (64.9 cm) and leaf blade mass (3,352.9 kg DM ha-1). The higher senescence rate of cv. Tamani (2.1 cm tiller-1 d-1) resulted in the highest percentage of dead material (1,815.5 kg ha-1) being found in the herbage mass of this cultivar. Cultivars Paiaguás, Marandu and Tamani were established at 44 d, whereas cv. Quênia and Ipyporã were established at 51 and 58 d after sowing, respectively, in the Brazilian Cerrado.
Key words: Morphogenesis, Megathyrsus maximus, Pasture, Urochloa brizantha.
Received: 10/08/2021
Accepted: 23/02/2022
Introduction
Forage plants are the main source of feed for ruminants in Brazil and contribute significantly to food production. In the Cerrado Biome, a relevant region for Brazilian livestock, Urochloa brizantha and Megathyrsus maximus genera are the most used due to their high yield potential and adaptability to the tropical climate(1,2). However, most cultivated areas are degraded or in the process of degradation, which has constituted a major obstacle to the expansion and intensification of animal production in grazing systems(3).
Proper establishment of forage species is fundamental for perenniality and productivity of a pasture(4). Establishment phase is a critical moment in the formation of pastures. Oftentimes, it represents the beginning of the degradation process or the implementation of a perennial and productive pasture, depending on the interaction between soil, plant and climate. Efficient soil correction and fertilization practices; the choosing the appropriate period for sowing the pasture; and the right moment for the first grazing are essential to ensure plant germination and growth(5).
Understanding the evolution of the pasture structure throughout the establishment period allows for greater assertiveness about the time for the first grazing to be performed. In addition, understanding the morphological and structural changes of forage cultivars when subjected to different soil-climatic conditions allows to identify their mechanisms of adaptation to the environment and helps in choosing those cultivars with more vigorous establishment(6).
In view of the above-described scenario, this study was undertaken to evaluate the time for the establishment of tropical forage grasses in the Cerrado Biome based on morphogenetic and structural traits.
Material and methods
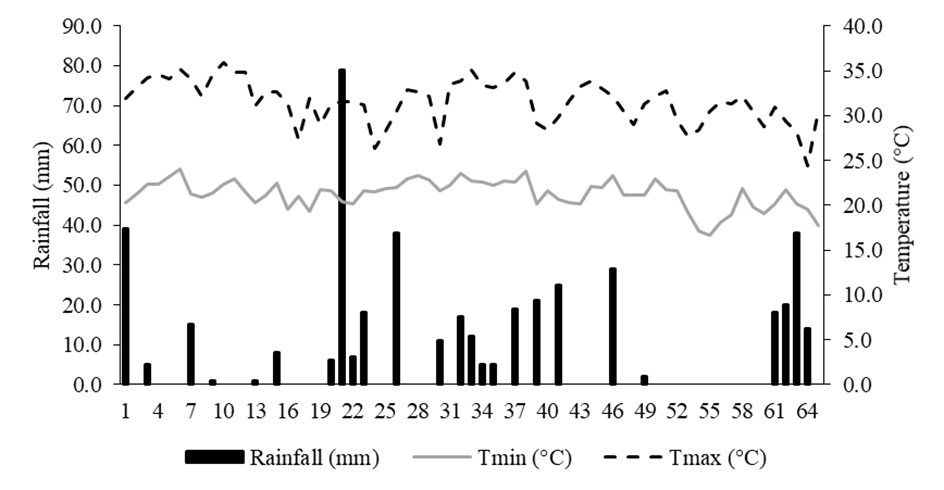
The experiment was carried out from December 15, 2020, to February 19, 2021, on the Fazenda Escola farm, of the Federal University of Mato Grosso do Sul, located in the municipality of Terenos - MS, Brazil (20°26'31" S, 54°51'36" W, 437 m asl). The climate of the region is classified as tropical rainy savanna (Aw subtype), characterized by the seasonal distribution of rainfall (Köppen). Temperature data were obtained from the INMET database and the precipitation data (Figure 1) were recorded from a rain gauge installed at the experiment site. The accumulated precipitation during the experimental period was 453 mm.
Figure 1: Rainfall and minimum (Tmin) and maximum (Tmax) temperatures during the experimental period
The soil in the experimental area is classified as a Red Oxisol with a very clayey texture(7). Before sowing, a soil sample was taken from the 0-20 cm layer for chemical analysis (Table 1). Based on these results, fertilization was carried out at sowing with 70.0 kg ha-1 of P2O5 and 35 kg ha-1 of K2O.
Table 1: Chemical characteristics of the soil in the experimental area, in the 0-20-cm depth layer
pH* | Ca2+ | Mg2+ | K+ | Al3+ | H+Al | SB | CEC | BS | OM | P |
5.9 | -------------------------------cmolc dm-3------------------------------- | -----%----- | mg dm-3 | |||||||
5.9 | 4.0 | 0.11 | - | 5.7 | 10.0 | 15.7 | 63.7 | 3.4 | 8.3 | |
*pH in water 1:2.5; SB: sum of bases (Ca + Mg + K); CEC: cation-exchange capacity at pH 7.0 [SB+(H+Al)]; BS: base saturation [(SB/CEC) * 100]; OM: organic matter.
The experimental design was a randomized blocks with five treatments and four replications. The treatments consisted of three Urochloa brizantha cultivars (Paiaguás, Ipyporã and Marandu) and two Megathyrsus maximus cultivars (Quênia and Tamani). The experimental area (3.14 ha) was divided in four blocks of 7,850 m2. Each block was composed of five plots measuring 1,570 m². The soil was mechanically prepared with deep plowing and level-disc harrowing. Sowing was carried out by broadcasting on December 15, 2020. The sowing rate was calculated as described by Dias-Filho(4), considering a seed value for cultivation of 60 % for the Urochloa brizantha cultivars and 40 % for the Megathyrsus maximus cultivars. A road roller was used to increase the soil-seed contact.
The evaluation spanned five weeks, from d 30 to d 65 after sowing. Morphogenetic and structural traits of the pastures were assessed at seven-day intervals. Canopy height (cm) was measured in 15 representative points per experimental plot, using a millimetric ruler. The canopy height at each point corresponded to the average height of the curvature of the leaves around the ruler.
Tiller density (TD, tillers m-2) was evaluated at three points per experimental plot, by counting all the tillers within a 0.50 × 0.50 m square frame (0.25 m2). The sampling points were fix throughout the experimental period, marked with wooden stakes.
Morphogenetic and structural traits of the forage canopy were evaluated using the tiller-tagging technique. Three tillers were marked per experimental plot using colored threads and measured weekly with a ruler graduated in centimeters. The heights of pseudostem and extended tiller and the length of each leaf were measured every seven days to estimate the following variables: leaf appearance rate (LAR, leaves tiller-1 d-1); phyllochron (days leaf-1 tiller-1); leaf elongation rate (LER, cm tiller-1 d-1); stem elongation rate (SER, cm tiller-1 d-1); final leaf length (FLL, cm tiller-1); leaf senescence rate (LSR, cm tiller-1 d-1); number of live leaves (NLL, leaves tiller-1); and leaf lifespan (LLS, d), as proposed by Lemaire and Chapman(8).
The cut to determine the forage mass and morphological components occurred when the canopy intercepted, 95.0 ± 3.5 % of the incident light, 65 d after sowing. The light intercepted by the canopy was estimated using a canopy analyzer (PAR Ceptometer - 80 AccuPAR Linear PAR / LAI; DECAGON Devices), at 15 random points per experimental unit. At each point, a reading was taken at the top of the forage canopy and another at 10 cm of the ground. Thus, at 65 d after sowing, the herbage dry mass (DM, kg DM ha-1 by cutting the herbage contained within three 1-m² squares per experimental plot. The samples were weighed and dried in a forced-air oven at 55 ºC until constant weight and then weighed again to determine the herbage dry mass. To evaluate the morphological components of the herbage, three sub-samples were extracted from the samples collected to determine HM. These were separated into leaf (leaf blade), stem (stem + sheath), dead material and undesirable plants. Leaf: stem ratio was calculated as the ratio between leaf blade dry mass (LBM, kg ha-1) and stem mass (SM, kg ha-1). Canopy height, TD and NLL data were subjected to analysis of variance, considering a randomized-block design with repeated measures over time. The effect of cultivars was allocated to the plot, and days after sowing (30, 37, 44, 51, 58 and 65 d) to the subplot (repeated measurements over time). The following model was used:
Yijk = μ + Ci + Bj + αij + Dk + CDik + βijk,
where:
Yijk= value observed in cultivar i, block j and day k;
μ= overall-mean effect;
Ci= effect of cultivar i;
Bj= effect of block j; αij : effect of random error attributed to the plot;
Dk= effect of day after sowing k;
CDik= interaction effect between cultivar and day;
βijk= random error attributed to the subplot.
When significant by the F-test, the cultivars were compared by Tukey’s test at a significance level of 5%, whereas the effect of days after sowing was analyzed using regression equations.
The remaining variables were subjected to analysis of variance according to the following model:
Yij = μ + Ci + Bj + αij,
where:
Yij= value observed in cultivar i and block j;
μ= overall-mean effect;
Ci= effect of cultivar i;
Bj= effect of block j; and
αij= random-error effect.
When significant by the F-test, the effects of the cultivars were analyzed by Tukey’s test at 5% significance. All statistical analyzes were performed using the MIXED procedure, in SAS ver. 9.1.
Results
The interaction cultivar by day after sowing was significative (P<0.05) for canopy height, TD and NLL. Canopy height increased linearly for all cultivars, with estimated daily increments of 1.52, 0.95, 1.21, 2.53 and 1.01 cm for Paiaguás, Ipyporã, Marandu, Quênia and Tamani, respectively (Table 2). On d 30 after sowing, cultivar Quênia had a greater height than cultivar Ipyporã, but there was no difference among the other cultivars. On d 37, the cultivar Quênia presented a higher canopy than the cultivars Ipyporã and Paiaguás. In the other periods, the cultivar Quênia had the largest canopy, followed by cvs. Marandu, Paiaguás and Tamani.
Tiller density increased linearly for Urochloa brizantha cultivars and decreased linearly for Megathyrsus maximus cultivars during the establishment period. The estimated increase in number of tillers in Paiaguás, Ipyporã and Marandu was 10.2, 10.8 and 6.6 tillers m-2 d-1, respectively. In Quênia and Tamani, the tiller population decreased by 5.09 and 29.67 tillers m-2 d-1, respectively. The TD at 30, 37, 44 and 51 d after sowing was higher in the Tamani cultivar. On the other hand, on day 58 after sowing, Tamani presented higher TD than Ipyporã, Marandu and Quênia, with no differences between the last two and Paiaguás. Finally, on d 65, the TD of the Tamani above that observed in Marandu and Quênia (Table 3).
Quênia maintained a constant NLL since the beginning of the evaluation period. In Marandu, there was an estimated daily increase of 0.05 leaves tiller-1. In the other cultivars, the NLL fitted a second degree-linear regression. Paiaguás and Ipyporã reached the maximum NLL at 42.5 and 47.0 d after sowing, respectively. Tamani reached an estimated minimum NLL at 58.3 d. 30 d after sowing, no differences were observed in the NLL between cultivars. At 37 d, a difference was found between the Paiaguás and Tamani. In the other periods (30, 44, 51, 58 and 65) the Urochloa brizantha cultivars presented a higher NLL (Table 4).
Leaf lifespan did not differ between the cultivars (Table 5). Ipyporã and Marandu showed higher LAR than Tamani, whereas Paiaguás and Quênia showed intermediate values. The phyllochron differed between Tamani and Ipyporã, which presented the highest and lowest values, respectively, while the cultivars Paiaguás, Marandu and Quênia presented intermediate values. The lowest LER were observed in Paiaguás, Ipyporã and Tamani, and the highest in Quênia, which in turn was above Marandu. As a result, Quênia showed the highest FLL. Paiaguás and Quênia had the highest SER. Lastly, LSR was highest in Quênia and lowest in Marandu, while Paiaguás, Ipyporã and Tamani showed intermediate values (Table 5).
Herbage mass did not differ between cultivars (Table 6). Quênia produced the largest LBM, whereas Paiaguás and Ipyporã showed the lowest, the others showed intermediate values. Stem mass was larger in Marandu and Quênia than in Ipyporã and Tamani. The SM of Paiaguás was similar to the others. Cultivar Tamani showed the largest dead material mass (DMM). The only difference for undesirable-plant mass (UPM) was found between Paiaguás and Tamani, whereas the Tamani pastures did not have UPM. The highest leaf:stem ratio occurred in Tamani, Ipyporã and Quênia.
Discussion
The linear increase in canopy height is consistent with the establishment period (Table 2), since when the forage is in the initial phase of vegetative growth, an increase in the average height of the sward over the days is observed, regardless of the cultivar(5,6). The recommended height to interrupt growth is around 30 cm for Brachiaria cultivars(9,10,11), 70 cm for Quênia(12) and 35 cm for Tamani(13). Paiaguás, Marandu and Tamani reached the height recommended for growth interruption by d 44 after sowing, whereas Quênia and Ipyporã reached this value at 51 and 58 d, respectively.
The high tiller population observed at all assessment periods in Quênia and Tamani may partly explain the reduction in DT (Table 3). Higher tiller populations during pasture vegetative growth promote greater intraspecific competition for light, reducing the amount and quality of light reaching the base of the canopy(14), which results in tiller mortality(15). Therefore, the time when the cultivars reached the recommended height to stop their growth would be adequate to carry out the first grazing
The number of live leaves differed between cultivars (Table 4). In Paiaguás and Ipyporã, there was an increase in NLL until it reached its maximum value, at 42.5 and 47.0 d after sowing, respectively. From that point, for each new leaf that appeared, another one started to die. However, the linear increase in NLL in cv. Marandu indicates that this cultivar did not reach the maximum value during its establishment. The always-higher NLL in Urochloa brizantha cultivars in comparison to Megathyrsus maximus cultivars can be attributed to their genetics, since the number of leaves formed in Urochloa brizantha cultivars is greater than in plants of Megathyrsus maximus(5,6,16).
Among the Megathyrsus maximus cultivars, Quênia maintained a constant NLL since the beginning of the evaluation period (30 days after sowing). On the other hand, in cv. Tamani, NLL decreased up to a minimum value that occurred on day 58 after sowing. Subsequently, there was an increase in the number of leaves per tiller. This behavior was due to the high TD of cv. Tamani, which promoted a compensatory effect, since, canopies with a high TD have shorter tillers, but these have low growth rates and vice-versa(17,18). This compensatory mechanism is evidenced by the morphogenetic variables evaluated during the establishment period, since Tamani was the one with fewest leaves, which in turn increased the phyllochron (Table 3).
For tropical pastures in a vegetative stage, morphogenesis can be described by LAR, LER, LLS(18) and SER(19). These traits are genetically determined and, as such, vary according to the evaluated genotype. Furthermore, variations in morphogenesis determine structural traits of the pasture(15,19). Therefore, for a consistent interpretation of these variables, the interactions between them must be taken into account.
The variations in LER reflected the morphological differences between the cultivars, especially in FLL, a genetically determined structural trait of the forage canopy(18). As described by Lemaire and Chapman(8), LER tends to follow the behavior of FLL. This association could be observed in cv. Quênia, which showed the highest values for both LER and FLL (Table 5). Marandu, Paiaguáis and Quênia showed greater stem elongation of all because these cultivars are taller (Table 2), which evidences the association between SER and the height of each cultivar(20).
The higher LSR observed in cv. Quênia can be attributed to its higher rates of leaf appearance and elongation. With new tissues growth, an increase of the rate of senescence of older tissues are expected, due to the plant renewal process(18). Cultivar Marandu had the lowest LSR, which can be explained because this cultivar did not reach the maximum NLL (Table 4), and senescence in forage plants is enhanced after the maximum NLL has emerged(19).
The similar HM between the cultivars may reflect the negative correlation between height and TD(21), which induces a compensation in HM. Cultivar Quênia had the highest values of rate of leaf appearance, elongation, and FLL. This combination of factors was responsible for the highest LBM occurring in this cultivar. Stem mass followed the trend observed for SER. Luna et al(16) reported a similar result, in which the stem accumulation rate behaved similarly to SER, regardless of the species evaluated.
The greater DMM contribution to the HM in cv. Tamami was a result of the high LSR and tiller mortality found in this cultivar (Tables 3 and 4). Due to its high TD, it is possible that Tamani would have quickly reached the critical leaf area index(22). Subsequent increases in leaf area index lead to reduced leaf accumulation and increased leaf and tiller mortality(23). On the other hand, this increased competition for light decreased the number of undesirable plants in the Tamani pastures.
These findings suggest that all cultivars exhibited vigorous establishment, given their high rates of tissue renewal, which promoted changes in the structural traits of the pasture(24). As a consequence, herbage mass, morphological composition and tiller population were modified.
Conclusions and implications
It was found that, depending on the morphogenetic and structural characteristics, the establishment time in the Cerrado Biome is 44 days after sowing for cvs. Paiaguás, Marandu and Tamani; and 51 and 58 days after sowing for cvs. Quênia and Ipyporã, respectively. Morphogenic traits are genetically determined and, as such, vary between genotypes; in addition, variations in morphogenesis determine structural characteristics of the pasture. Evaluating the morphogenetic and structural variables simultaneously allows observing the dynamics of tissue emergence and death both within a tiller and for the entire tiller population. Thus, allowing greater precision at the ideal time to stop the growth of grasses.
Acknowledgments
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Thanks also to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Apoio ao Desenvolvimento da Educação, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) and the Universidade Federal de Mato Grosso do Sul for their support.
Literature cited:
Table 2: Canopy height (cm) of tropical forage grasses in different evaluation periods during establishment in the Brazilian Cerrado biome
Cultivar | Days after sowing | P-value | Regression equation | R2 (%) | |||||||
30 | 37 | 44 | 51 | 58 | 65 | L | Q | ||||
Paiaguás | 12.1ab | 16.0b | 27.9b | 40.1cb | 47.2cb | 60.3b | 0.001 | 0.522 | Y = -33.30 + 1.42x | 98.5 | |
Ipyporã | 8.5b | 12.8b | 20.5b | 26.8c | 30.6c | 43.0c | 0.001 | 0.554 | Y = -21.23 + 0.95x | 97.6 | |
Marandu | 17.6ab | 24.8ab | 33.4b | 44.2b | 51.5b | 58.6cb | 0.001 | 0.896 | Y = -19.09 + 1.21x | 99.6 | |
Quênia | 28.5a | 37.0a | 57.3ª | 69.5ª | 87.1ª | 119.7a | 0.001 | 0.101 | Y = -53.45 + 2.53x | 96.5 | |
Tamani | 19.5ab | 24.1ab | 35.8b | 40.1cb | 42.5cb | 57.1cb | 0.001 | 0.765 | Y = -11.56 + 1.01x | 95.5 | |
L= linear; Q= quadratic. Y is the dependent variable and X is the independent variable (days after sowing).
abc Lowercase letters in the same column differ from each other by Tukey’s test (P<0.05); Standard error of the mean= 4.38;
Table 3: Tiller density (tillers m-2) of tropical forage grasses in different periods of evaluation during establishment in the Brazilian Cerrado biome
Cultivar | Days after sowing | P-value | Regression equation | R2 (%) | |||||||
30 | 37 | 44 | 51 | 58 | 65 | L | Q | ||||
Paiaguás | 210.0c | 245.7c | 318.0b | 412.3b | 487.0ab | 546.0ab | 0.001 | 0.821 | Y = -114.50 + 10.20x | 98.8 | |
Ipyporã | 166.0c | 229.0c | 314.0b | 396.7b | 445.7b | 546.7ab | 0.001 | 0.934 | Y = -161.46 + 10.76x | 99.5 | |
Marandu | 221.0c | 257.7c | 354.3b | 422.7b | 445.7b | 417.7b | 0.002 | 0.270 | Y = 39.93 + 6.59x | 84.5 | |
Quênia | 602.0b | 583.6b | 531.7b | 435.0b | 456.5b | 448.2b | 0.016 | 0.538 | Y = 751.27 - 5.09x | 83.3 | |
Tamani | 1534.4a | 1605.0a | 1617.3a | 864.4a | 737.2a | 742.0a | 0.001 | 0.136 | Y = 2594.20 - 29.67x | 77.3 | |
L= linear; Q= quadratic. Y is the dependent variable and X is the independent variable (days after sowing).
abc Lowercase letters in the same column differ from each other by Tukey’s test (P<0.05); Standard error of the mean= 63.92;
Table 4: Number of live leaves per tiller in tropical forage grasses in different periods of evaluation during establishment in the Brazilian Cerrado biome
Cultivar | Days after sowing | P-value | Regression equation | R2 (%) | ||||||||
30 | 37 | 44 | 51 | 58 | 65 | L | Q | |||||
Paiaguás | 5.3a | 6.3a | 7.5a | 6.0a | 5.1ab | 5.7ab | 0.227 | 0.002 | Y = -1.26 + 0.34x - 0.004x2 | 39.0 | ||
Ipyporã | 4.0a | 5.6ab | 6.6ab | 6.1a | 5.4ab | 5.9ab | 0.105 | 0.001 | Y = -5.61 + 0.47x - 0.005x2 | 71.8 | ||
Marandu | 4.9a | 5.2ab | 6.0ab | 6.4a | 6.6a | 6.3a | 0.001 | 0.117 | Y = 3.61 + 0.05x | 81.7 | ||
Quênia | 4.5a | 5.2ab | 5.2b | 4.3b | 4.5b | 4.5b | 0.367 | 0.326 | Y = 4.7 | - | ||
Tamani | 4.9a | 4.5b | 3.8c | 2.8b | 2.8c | 3.5c | 0.081 | 0.008 | Y = 12.87 - 0.35x + 0.003x2 | 86.5 | ||
L= linear; Q= quadratic. Y is the dependent variable and X is the independent variable (days after sowing).
ab Lowercase letters in the same column differ from each other by Tukey’s test (P<0.05), Standard error of the mean= 0.40;
Table 5: Structural and morphogenetic traits of tropical forage grasses during the establishment period in the Brazilian Cerrado biome
Variable | Cultivar | SEM | P-value | ||||
Paiaguás | Ipyporã | Marandu | Quênia | Tamani | |||
LAR, leaves tiller-1 d-1 | 0.16ab | 0.18a | 0.17a | 0.15ab | 0.13b | 0.01 | 0.0181 |
Phyllochron, days leaf-1 tiller-1 | 6.4ab | 5.7b | 5.8ab | 6.9ab | 8.1a | 0.50 | 0.0355 |
LER, cm tiller-1 d-1 | 5.4c | 5.3c | 7.8b | 10.8a | 5.4c | 0.48 | 0.0001 |
SER, cm tiller-1 d-1 | 1.25a | 0.52b | 1.1a | 1.0a | 0.19b | 0.08 | 0.0001 |
LSR, cm tiller-1 d-1 | 1.7ab | 1.5ab | 1.2b | 2.5a | 2.1ab | 0.26 | 0.0312 |
FLL, cm | 30.2b | 29.1b | 43.1b | 64.9a | 36.0b | 3.50 | 0.0001 |
LLS, days | 37.9 | 32.0 | 34.4 | 32.7 | 28.2 | 2.40 | 0.2026 |
LAR= leaf appearance rate; LER= leaf elongation rate; SER= stem elongation rate; LSR= leaf senescence rate; FLL= final leaf length; LLS= leaf lifespan; SEM= standard error of the mean.
abc Lowercase letters in the same row differ from each other by Tukey’s test (P<0.05).
Table 6: Structural traits of tropical forage grasses during the establishment period in the Brazilian Cerrado biome
Variable | Cultivar | SEM | P-value | ||||
Paiaguás | Ipyporã | Marandu | Quênia | Tamani | |||
HM, kg DM ha-1 | 5405.0 | 4736.5 | 5766.5 | 6884.8 | 6007.5 | 529.5 | 0.1554 |
LBM, kg DM ha-1 | 1654.6b | 1962.7b | 2533.1ab | 3352.8a | 2571.7ab | 258.1 | 0.0131 |
SM, kg DM ha-1 | 1879.9ab | 1427.1b | 2345.7a | 2429.6a | 1619.3b | 209.2 | 0.0353 |
DMM, kg DM ha-1 | 546.7b | 449.2b | 484.3b | 839.4b | 1816.5a | 96.1 | 0.0001 |
UPM, kg DM ha-1 | 1444.9a | 937.8ab | 403.4ab | 263.0ab | 0.0b | 279 | 0.0378 |
Leaf:stem ratio | 0.9b | 1.5a | 1.1b | 1.4a | 1.59a | 0.1 | 0.0436 |
HM= herbage mass; LBM= leaf blade mass; SM= stem mass; DMM= dead material mass; UPM= undesirable-plant mass; SEM= standard error of the mean.
ab Lowercase letters in the same row differ from each other by Tukey’s test (P<0.05).